What you really NEED to know:
- Bromine is a more effective disinfectant in water than chlorine when the pH is greater than 8 (which is the case in many cooling waters)
- Chlorine and bromine are from the same chemical family of ‘oxidisers’, they disinfect by oxidising (chemically degrading) microbes. As oxidisers they are also corrosive to many metals.
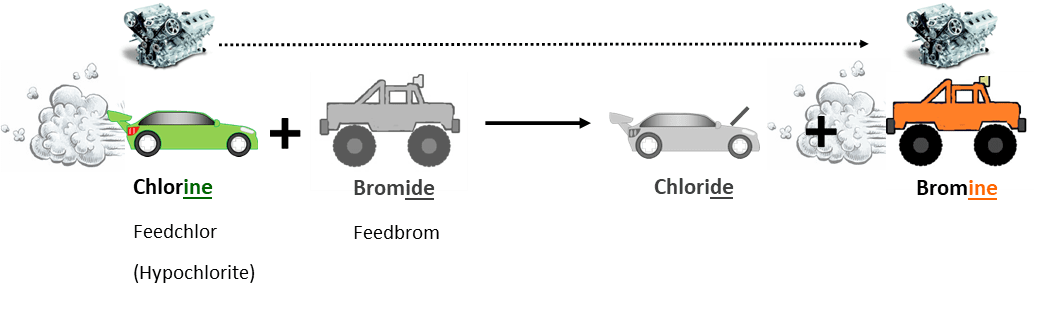
- Bromine can be generated at low concentrations in water by mixing active chlorine with a relatively harmless salt of bromine, called bromide. Bromide is the main ingredient of Feedbrom, we sometimes call it a ‘bromine donor’. Mixing Feedbrom (bromide) with Feedchlor (chlorine) in water generates ‘bromine’. We call this ‘in situ’ generation, because the bromine is created at the point of use (within the system) for safety reasons.
Bromine disinfection in cooling systems – why is it better than chlorine?
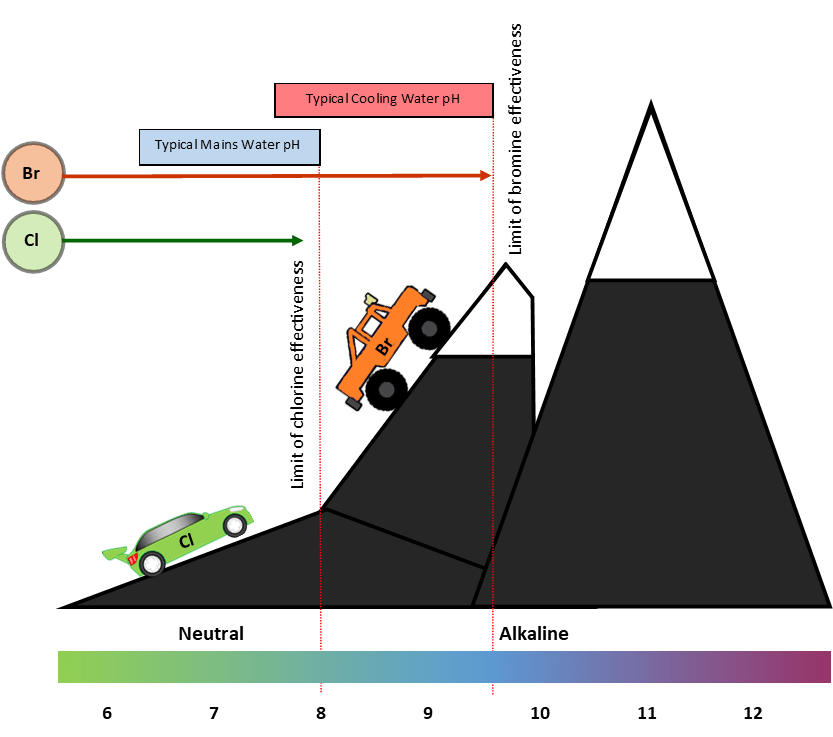
Because with increasing pH (typical in cooling system), bromine is a workhorse—it continues to work after chlorine has given up
Imagine chlorine and bromine as cars—both with the same powerful engine, except that chlorine is a sports car, and bromine is a 4×4.
Imagine the pH scale as a road which becomes increasingly steep and rocky as the pH climbs higher into the alkaline side (above 7).
The chlorine (sports car) gets as far as pH 8 before it gets too difficult, however, the bromine (4×4) can go as far as pH 9.5 before suffering a similar fate.
Chlorine gives up at pH 8; bromine will continue to work up to pH 9.5

Bromine generation in cooling systems
Sticking with the car analogy, here’s what happens when we generate bromine in a cooling system using Feedchlor and Feedbrom: